Frequently asked questions
Minimal residual disease (MRD) testing identifies the presence of tiny numbers of cancer cells that may remain in the body after treatment. Some MRD tests detect DNA fragments released by cancer cells into the bloodstream. These DNA fragments, known as circulating tumor DNA (ctDNA), are identified by sequencing the DNA in a blood sample.
This information can be used for the following purposes:
- To determine a patient’s risk of recurrence in the absence of additional treatment
- To identify patients who are more likely to benefit from additional treatment
- To monitor a patient’s disease while on treatment
- To identify patients with disease recurrence following definitive treatment
MRD testing can detect residual, recurrent, or resistant cancer cells earlier than traditional detection methods. Earlier detection enables treatment decisions to be made when the tumor burden is low, increasing the chances for successful treatment.
Searching for and analyzing ctDNA is a noninvasive way to test for residual disease. A liquid (blood-based) biopsy can detect the presence of tumor cells up to 6 months earlier than imaging, increasing the likelihood that a therapeutic intervention will be successful.
Haystack MRD is a liquid biopsy (blood) test that uses a tumor-informed approach to maximize the sensitivity and specificity of circulating tumor DNA (ctDNA) detection. Haystack MRD testing begins with whole-exome sequencing (WES) of a patient’s tumor tissue and a matched normal blood sample to identify patient-specific somatic mutations. A personalized mutation panel is then designed for each patient, and a next-generation sequencing (NGS)-based assay is performed to detect and monitor ctDNA in plasma to identify the presence of residual, recurrent, or resistant disease.
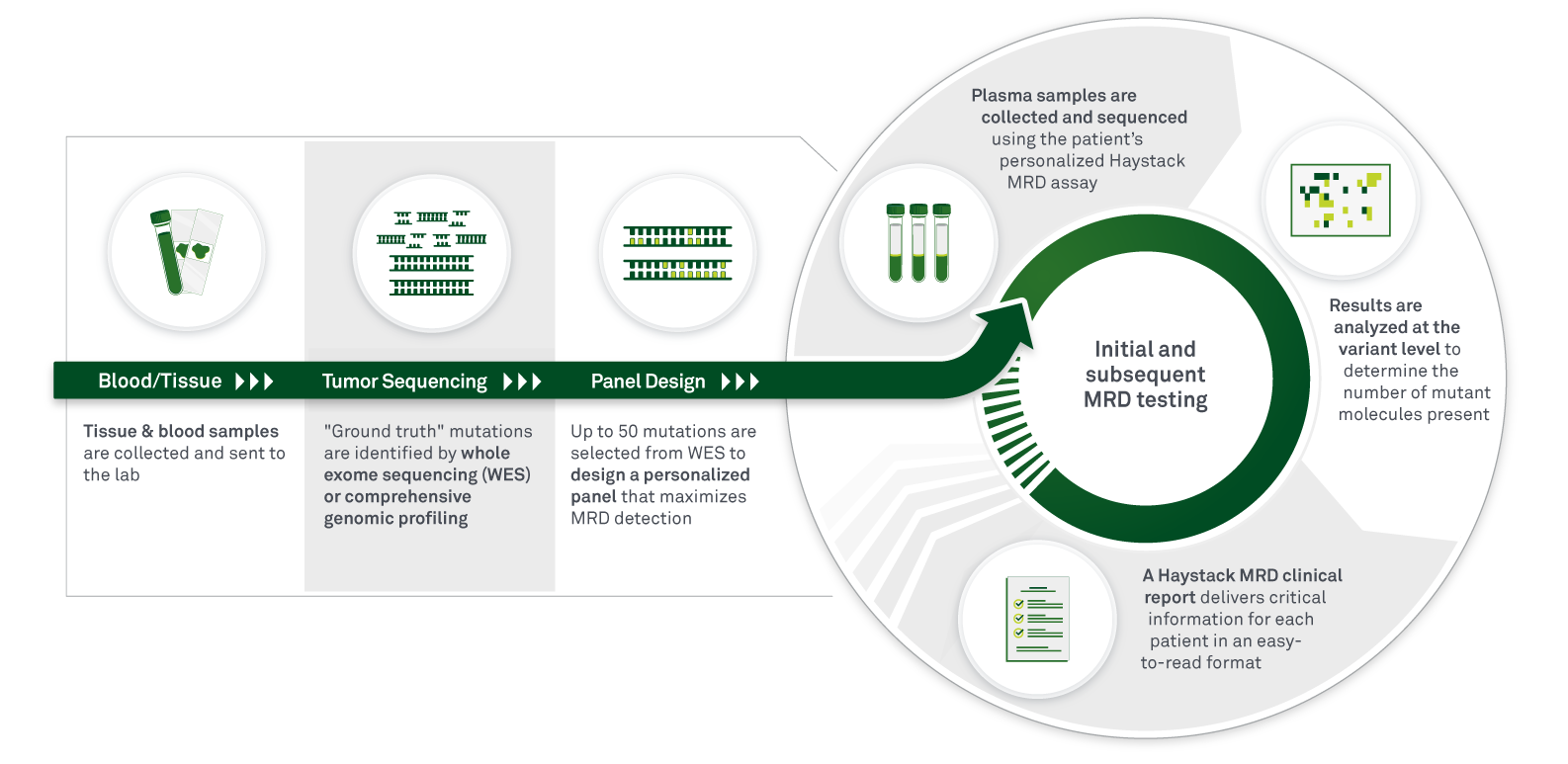
Haystack MRD is the only test specifically built with the sensitivity to accurately detect MRD in patients with early-stage disease. Haystack MRD detects ultralow levels of ctDNA to provide insights at critical moments for important treatment decisions, to accurately monitor treatment response and disease clearance, and to detect the earliest stages of disease relapse. Importantly, Haystack MRD’s superior performance ensures confidence in results and enables an early window of opportunity when clinical management decisions can have the greatest impact on patient outcomes.
Haystack MRD can greatly accelerate clinical development by enriching for a greater number of patients with residual disease than other tests can. By providing ultrahigh sensitivity, Haystack MRD is capable of identifying a larger pool of MRD-positive patients, which can significantly impact enrollment and time to trial completion. In addition, Haystack MRD can be used for monitoring treatment response for clinical investigations, facilitating early determination of treatment efficacy.
Haystack MRD will be broadly available, as patient samples can be provided to any of the more than 2,000 Quest Diagnostics Patient Service Centers across the US.
These key features differentiate Haystack MRD from other tests:
- Ultrahigh sensitivity for detection of MRD, therapeutic response monitoring, and surveillance for cancer recurrence—Haystack Duo™, the chemistry behind Haystack MRD, was developed to significantly reduce background noise to detect even the least abundant ctDNA molecules in blood, delivering industry-leading sensitivity with the ability to detect one mutant DNA molecule in a million normal DNA molecules. As a result, extremely low levels of ctDNA can be called with confidence.
- Tumor-informed, personalized assay—A personalized assay is developed based on “ground-truth” mutations that are identified via whole-exome sequencing (WES) in the original tumor sample.
- Built on decades of ctDNA clinical technology development—Haystack MRD was built from the ground up by pioneers in liquid biopsy technology to overcome the unique challenges of ctDNA detection in the MRD setting and does not rely on methods adapted from other intended uses such as NIPT (noninvasive prenatal testing) and tumor profiling of metastatic cancers.
- Optimal number of mutations tracked—With other tests, every mutation tracked increases the level of background signal that the test must deal with to try to discern tumor signal. Haystack’s proprietary technology effectively eliminates the background signal so that mutations are accurately tracked and the optimal number of mutations can be included in each patient’s test panel.
- Demonstrated clinical utility—Haystack MRD is the first method to generate interventional data supporting the clinical utility of using ctDNA MRD testing to guide adjuvant therapy decisions (see “What is the DYNAMIC study?” below).
We are working with Centers for Medicare & Medicaid Services (CMS) and private insurance companies to acquire coverage for Haystack MRD upon commercial launch.
Yes, our platform can be used to help stratify patients for enrollment in adjuvant-setting and second-line therapy trials and to monitor therapeutic efficacy, providing significant time and cost savings.
The test will launch commercially in 2024. For information about potential early access partnerships, contact our commercial team at info@haystackoncology.com.
To design the initial, personalized assay, Haystack MRD requires a formalin-fixed, paraffin-embedded tumor sample (or in some cases, a biopsy sample) and a blood draw. For monitoring MRD status at all future time points, a blood draw is required.
To build the personalized MRD panel, a tissue sample of at least 25 mm2 of surface area and 50 µm of depth are required. Biopsies are accepted, but they may have a higher failure rate if they yield fewer non-necrotic tumor cells. A block with 4–5 needle biopsies will typically yield enough DNA for successful sequencing.
The tumor sample is obtained surgically (typically when a tumor is removed surgically from the body with curative intent) and should be formalin-fixed and paraffin-embedded. When Haystack MRD is ordered, we will work with the pathology lab to obtain the tumor tissue.
Results for an initial order can take up to 4 weeks, which includes time to build a personalized test for new patients. Any subsequent test results will be provided within 5–7 days after the order is placed.
Our proprietary technology effectively eliminates the background signal so that the optimal number of mutations can be included in each patient’s test panel. Haystack MRD tracks up to 50 mutations, prioritizing truncal somatic mutations.
Yes, all Haystack MRD testing is done in a CLIA-certified lab.
A whole-exome sequencing report will not be available. The data from WES will be fed into Haystack MRD’s mutation-selecting algorithm to create a personalized MRD test.
If an additional paraffin block from the patient’s tumor is available, we can attempt to rescue failed tumor whole-exome sequencing (WES). To reduce the risk of failures, we ask that the block with the highest tumor cellularity and lowest amount of necrotic tissue is selected.
Cell-free DNA (cfDNA) is released into the bloodstream from various cells in the body, especially those of the hematopoietic system. If a tumor is present in the body, cells from the tumor may also release DNA into the bloodstream. This circulating tumor DNA (ctDNA) typically makes up a small proportion of the total cfDNA in the bloodstream.
Haystack Duo is the name of the chemistry that powers Haystack MRD. While the details of the chemistry are proprietary, Duo is a highly optimized version of Safe-SeqS chemistry, which was employed in the DYNAMIC study (see “What is the DYNAMIC study?” below). Duo is the only chemistry that was purpose-built and specifically optimized to detect the ultralow ctDNA levels that are found in postsurgical patients with residual or recurrent disease.
The DYNAMIC study, reported at the 2022 American Society of Clinical Oncology (ASCO) meeting and published in the New England Journal of Medicine, is the first prospective, interventional, randomized trial to demonstrate the clinical utility of a ctDNA-guided treatment strategy. The study evaluated the potential of ctDNA-based MRD testing to guide adjuvant therapy decisions in stage II colorectal cancer patients who had undergone curative-intent surgery. The results of the study showed that a ctDNA-guided treatment strategy led to 50% fewer patients receiving chemotherapy as compared to patients treated based on standard-of-care clinicopathological risk factors, with no reduction in 2-year recurrence-free survival. Read more about the DYNAMIC study here.
News and events

American Association for Cancer Research 2024
Haystack MRD™ by Quest Diagnostics® will be attending the 2024 American Association for Cancer Research AACR annual meeting taking place April 5 10 2024 in San Diego CA The AACR annual meeting stands as the cornerstone of the cancer research

Haystack Oncology, developer of Haystack MRD™, and TriSalus Life Sciences collaborate in connection with the clinical development of TriSalus’ TLR9 agonist in hepatocellular carcinoma, intrahepatic cholangiocarcinoma, and pancreatic adenocarcinoma
Haystack Oncology a Quest Diagnostics NYSE DGX company and developer of best in class personalized MRD technology Haystack MRD™ has entered a research collaboration with TriSalus Life Sciences to evaluate therapeutic response and provide molecular insights in connection with the

Haystack Oncology and Alliance Foundation Trials collaborate to use Haystack MRD™ technology in phase II clinical trial for unresectable stage III NSCLC
Haystack Oncology a Quest Diagnostics NYSE DGX company has entered into a collaboration with Alliance Foundation Trials LLC AFT for research use of Haystack Oncology 8217 s personalized MRD technology Haystack MRD™ to analyze therapeutic response and provide molecular insights