The landmark DYNAMIC study is the first prospective, randomized, interventional trial to demonstrate the clinical benefit of MRD testing to guide adjuvant therapy
An enduring challenge in cancer management is the accurate identification of patients who harbor any remaining cancer cells—or minimal residual disease (MRD)—following curative-intent treatment such as surgery to remove a tumor. These patients require additional treatment to eradicate MRD and decrease the risk of a later recurrence. The emergence of MRD testing via ultra-sensitive circulating tumor DNA (ctDNA) detection directly addresses this challenge and has the potential to revolutionize the clinical management of cancer patients.
The DYNAMIC study, reported at the 2022 American Society of Clinical Oncology (ASCO) meeting and published in the New England Journal of Medicine, is the first prospective, interventional, randomized trial to demonstrate the clinical utility of a ctDNA-guided treatment strategy.
For patients with stage II colon cancer, curative-intent surgery is performed according to the current standard of care. However, some patients have a higher risk of recurrence after surgery and may benefit from adjuvant chemotherapy, a post-surgical treatment to remove any residual cancer cells. The challenge for clinicians is to discern which patients require adjuvant therapy versus those who do not. Traditionally, clinicopathologic features have served as the basis for patient risk stratification to guide clinicians in making adjuvant chemotherapy decisions. However, this strategy has been shown to be highly imprecise in defining an individual patient’s risk of recurrence, leading to both overtreatment, which results in unnecessary clinical toxicity and financial burden, and undertreatment, which can increase risk of cancer recurrence, for a significant proportion of high-risk patients. Therefore, there is an urgent need for more precise and personalized information to determine a patient’s risk of recurrence following curative-intent surgery for better adjuvant therapy decision-making.
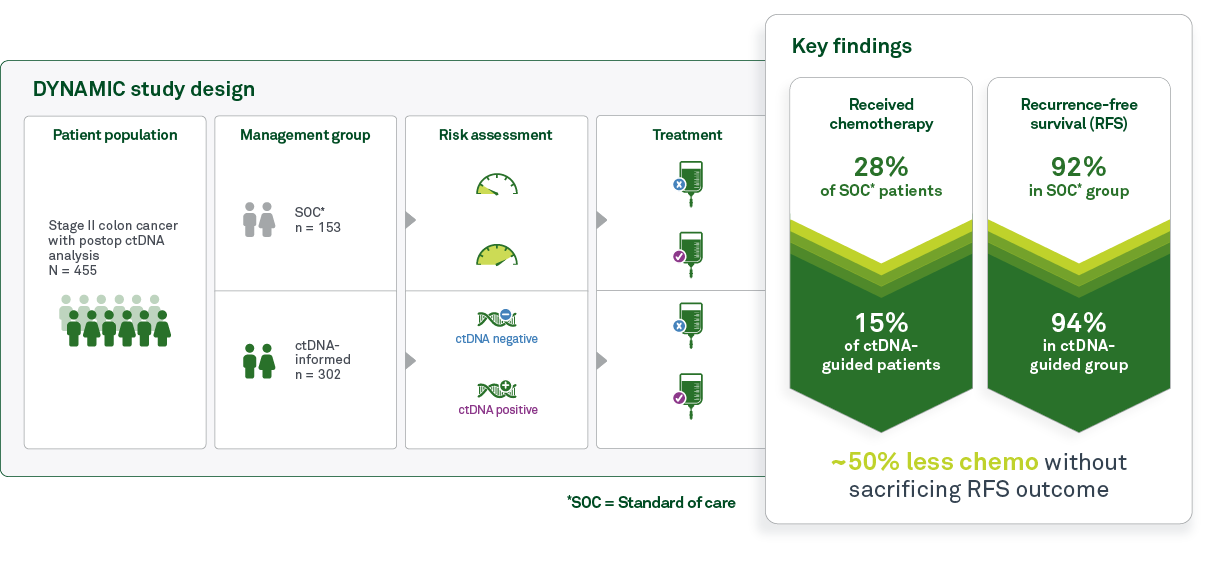
DYNAMIC study design
The DYNAMIC trial explored the potential of ctDNA to guide adjuvant therapy decisions in patients with stage II colon cancer who had undergone curative-intent surgery. The study involved 455 patients who were randomized in a 2-to-1 ratio to be treated according to ctDNA results (“ctDNA-guided group”) or according to standard of care clinicopathological features (“standard of care group”).
Patients in the ctDNA-guided group were treated solely based on the results of ctDNA testing from blood samples collected four and seven weeks post-surgery. Patients with a positive ctDNA result at either time point were given adjuvant chemotherapy, whereas patients without ctDNA detected did not receive chemotherapy. In contrast, patients in the standard of care group received adjuvant chemotherapy based on traditional risk assessment using clinicopathological features such as tumor size and lymph node involvement.
DYNAMIC study results
Chemotherapy usage was halved in the ctDNA-guided group versus the standard of care group (15% of patients received therapy versus 28%, respectively) with no impact to 2-year recurrence-free survival. These findings are consistent with previous observations of overtreatment for stage II CRC and support the utility of ctDNA to better inform adjuvant therapy decisions.
Beyond stage II colorectal cancer
Prior to the DYNAMIC study, a wealth of observational data revealed that ctDNA is highly predictive of disease recurrence. While these studies were important in establishing the prognostic value of ctDNA, the clinical utility of ctDNA testing to inform real-time patient management was not definitively established until the DYNAMIC trial. Additional studies are exploring the utility of ctDNA to elucidate clinical decisions across other indications beyond stage II colon cancer:
- For stage III CRC, it is generally well accepted that all patients should receive adjuvant therapy; however, the appropriate treatment intensity and duration may be sub-optimal when based on clinicopathologic risk factors alone. The DYNAMIC-III study is currently investigating the utility of ctDNA to guide therapy de-escalation and escalation to reduce over- and under-treatment, for stage III CRC patients.
- Ovarian cancer is an aggressive disease that is often not diagnosed until later stages. Patients with ovarian cancer may receive neoadjuvant (prior to surgery) therapy, followed by de-bulking surgery and adjuvant therapy. The DYNAMIC-ovarian study is exploring ctDNA as a prognostic marker when evaluated before therapy initiation, while on treatment, and at the end of treatment, in both the neoadjuvant and adjuvant settings.
- Additional studies are investigating the validity and utility of ctDNA testing performed for patients with rectal and pancreatic cancer.
Haystack MRD: Powered by foundational clinical utility
Haystack Duo™ sequencing chemistry, which powers Haystack MRD, is an enhanced version of the method used for ctDNA detection across the family of DYNAMIC clinical studies. Duo was specifically designed for tumor-informed, personalized MRD testing, and achieves greater sensitivity by reducing technical noise >100x compared to other methods, enabling detection of ctDNA at the lowest levels of residual disease.
Haystack MRD provides robust MRD detection at specific, individual time points that correspond to therapeutic “windows of opportunity” when clinical decisions can be made that will improve patient outcomes. This powerful, early detection is essential for clinical utility of an MRD test and contrasts with less-sensitive methods that combine multiple tests over time, often not detecting positive patients until after the window of opportunity has closed.